Details of the Drug
General Information of Drug (ID: DMD7X1O)
| Drug Name |
Dexrazoxane
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
24584-09-6; Zinecard; (S)-4,4'-(Propane-1,2-diyl)bis(piperazine-2,6-dione); Cardioxane; ICRF-187; Dexrazoxano; Dexrazoxanum; Dextrorazoxane; Dexrazoxanum [INN-Latin]; Dexrazoxano [INN-Spanish]; Desrazoxane; Eucardion; ADR 529; ICRF 187; (+)-(S)-4,4'-Propylenedi-2,6-piperazinedione; Dexrazone; ADR-529; (+)-1,2-Bis(3,5-dioxo-1-piperazinyl)propane; HSDB 7319; UNII-048L81261F; NSC169780; dyzoxane; BRN 5759131; CHEBI:50223; 4-[(2S)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione; NSC 169780; AK-72797; Razoxanum; Cardioxane; Dyzoxane; Savene; TopoTect; Totect; Dexrazoxane HCl; Dexrazoxane hydrochloride; ICRF 187 hydrochloride; Cardioxane (TN); Dexrazoxane (TN); Totect (TN); Zinecard (TN); Dexrazoxane (USAN/INN); Dexrazoxane [USAN:BAN:INN]; Soluble ICRF (L-isosomer); Razoxane, (S)-Isomer; Totect, ICRF-187, Zinecard, Cardioxane, Dexrazoxane Hydrochloride;(+)-(S)-4,4'-Propylenedi-2,6-piperazinedione; (+)-1,2-Bis(3,5-dioxopiperazin-1-yl)propane; (S)-(+)-1,2-Bis(3,5-dioxopiperazin-1-yl)propane; 2,6-Piperazinedione, 4,4'-(1-methyl-1,2-ethanediyl)bis-, (+)-(9CI); 4,4'-(2S)-propane-1,2-diyldipiperazine-2,6-dione; 4-[(2S)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione hydrochloride; Icrf-187
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
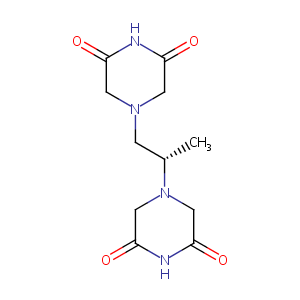 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 268.27 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.4 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Dexrazoxane (Comorbidity)
|
|||||||||||||||||||||||||||||
References
